Hydroxyl Functional Group Properties and Structures
Introduction
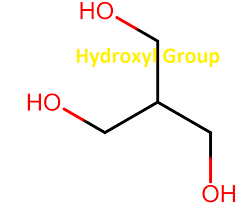
The hydroxyl functional group is the simplest of all the common organic functional groups. It consists of a hydrogen atom attached to an oxygen atom. It has two chemical properties: acidity, measured by its pH value, and nucleophilicity, or how reactive it is with other molecules. This post will focus on the properties and structures associated with this simple but essential part of biochemistry.
However, should you choose to skip this guide due to reasons such as a busy schedule, our professional writers for hire are ready to cover you by acing that assignment for you. Just place an order with us!
What Are Hydroxyl Groups?
They are chemical groups found in organic compounds on the carbon that is attached to oxygen. It has two lone pairs of electrons, making it possible to engage in hydrogen bonding. Water or ethanol are typical examples of hydroxyl group-containing substances.
There are three major classifications for this chemical functionality: alkoxy (RO), phenoxy (R-O-Ph), and carboxylate (RCO 2).
Hydroxyl functional group [H-OH] and Hydroxyl group (OH) are chemical functional groups. [H-OH] is derived from Hydrogen (H) and Oxygen (O). It can be found in compounds that contain one or more hydroxyl groups. The general formula of these compounds is represented as H-OH and its derivatives.

What Are the 7 Functional Groups?
The seven (7) groups are the hydroxyl group, the carbonyl group, the carboxylic acid group, the amine group, the amino group or amino acids, aldehydes, and ketones.
The carbonyl groups have an atom bonded to four different groups: a C=O double bond and three other organic groups. The carboxylic acid has the structure R-COOH. R represents a hydrogen atom or an organic group. The amine group is very similar to the hydroxyl group because it has one nitrogen, two carbons, and three other organic groups. However, there is no oxygen, and a CH3 group replaces the hydrogen atom. Aldehydes are organic groups with a carbonyl double bond attached to an alkyne (a C-C triple bond).


What is the Functional Group of Hydroxyl?
The functional group of hydroxyl is the -OH group. This group belongs to the family of polar uncharged organic groups.
Their polarity makes these oxygen-containing compounds good in aqueous solvents, such as water and many oils. These -OH groups are weak acids, either in solution or as part of the molecule, and can react with non-metals such as carbon.
Hydroxyl Reactive Functional Group
The reactive hydroxyl group is a chemical functionality involved in most reactions with other molecules or atoms. Hydrogen bonding is essential because it aids in solubility and structure. The presence of hydrogen bonds between two molecules is a crucial factor in influencing the solubility of one compound in another. Hydrogen bonding also gives rise to high boiling points and melting points for substances that contain it.
The general structure of this group is R-O-(H-OH). Some examples are alcohols, phenols, amines, carboxylic acids, esters, ethers, anhydrides, and various derivatives.
Hydroxyl Functional Group Properties
The hydroxyl group is polar, electrophilic, and nucleophilic. The polarity makes it a suitable hydrogen bond donor since water has a high affinity to this group. It can be found in many ions or salts, such as sodium hydroxide (NaOH), mercury (II) chloride (HgCl 2), and calcium hypochlorite (Ca(OCl)2).
Hydroxyl Functional Group Structure
The hydroxyl group [H-OH] comprises two hydrogen atoms bonded to a carbon atom with one oxygen attached. Hydroxyl compounds have an H-OH or R–OH formula, where the oxygen is written without brackets since it forms a bond to only one hydrogen atom. The –OH group is attached to the rest of the molecule along the carbon chain.
This functionality has two attachment sites in functional groups like ethers, so the location is specified as O-A-H and A-H.
You may be interested in Nitrogenous Waste Examples
Hydroxyl Functional Group Uses
In organic chemistry, hydroxyl groups are used to link different chains together in many types of molecules. They are also widely used in biochemical synthesis to create water-soluble compounds. Many pharmaceuticals use this functionality due to its ability to form hydrogen bonds. Hydroxyl groups are also used in detergents and fire retardants.
Hydroxyl Functional Group Safety Concerns
The [H-OH] is reactive due to this pair of nonbonding electrons that surround the oxygen atom. It has been modified by adding a carbon chain or group of particles around it to prevent unwanted reactions. This modification is called etherification because an oxygen atom has been replaced with an oxygen-containing group.
[H-OH] are organic compounds that are important in the biochemistry and physiology of almost all living things, most notably as water (H2O), but also including as alcohols, carboxylic acids, aldehydes, ketones, primary amines, and phenols.
Hydroxyl groups also commonly appear as components of larger molecules: glycols, phosphates, alcohols (ethanol), fats (triacylglycerol), and waxes contain the hydroxyl group attached to different atoms. The biochemical reactions that make use of these groups involve adding or removing a hydroxyl group from the substrate molecules.
The hydroxyl group comprises a hydrogen atom connected to a carbon atom which is in turn bonded to an oxygen atom: −OH, C-H-O−
[H-OH] on a molecule can be found in more complex molecules such as biochemicals. In these cases, the hydroxyl group is most commonly positioned at a carbon atom adjacent to a functional group such as an amine (NH 2 ) or carboxylic acid (-RCOOH). In organic synthesis, hydroxyl groups are used in reactions involving the elimination of water. They are called “aqueous work” [3] or, more descriptively, oxyhydrogen.


How Do Hydroxyl Groups Differ From One Compound to Another?
The difference between the various hydroxyl-containing compounds depends on their position and the rest of the molecule. Alcohol contains an -OH group bonded to carbon, while in acids, it is connected to an oxygen atom. For instance, ethanoic CH3COOH is an acid, and ethanol CH3CH2OH is alcohol.
What Are the Health Effects of Hydroxyl Group?
In general, the Hydroxyl group is a good thing for living things since it is a part of living cells. However, it can also be harmful. For instance, when a large amount of alcohol is consumed, it can cause health problems such as liver malfunction and anemia, or even death.
What is the Polarity of Hydroxyl?
In general, alcohols are less acidic than carboxylic acids, and they can act as solvents in many cases. These properties mainly depend on the rest of the molecule. For example, in alcohols, the -OH group is bonded to carbon, while in acids, it is connected to an oxygen atom.
For instance, ethanoic (CH3COOH) is an acid, and ethanol (CH3CH2OH) is alcohol. The polarity of the hydroxyl group depends on its environment or the location in which it resides. It can also be affected by what species are bonded to it.
How Do You Identify Hydroxyl Group in a Structure?
An organic compound can have more than one type of functional group on it. Their identification is made using either spectroscopy or chromatography.
Spectroscopic methods include infrared spectroscopy (IR), ultraviolet-visible spectroscopy (UV/VIS), and nuclear magnetic resonance (NMR). IR measures the absorption of infrared radiation at wavelengths that correspond to specific groups. UV/Vis spectroscopy measures the absorption of light in the ultraviolet-visible portion of the spectrum, which again corresponds to particular groups. NMR is used to differentiate between similar functional groups based on how they behave under carefully controlled magnetic fields and radio frequencies.
Chromatography techniques are used for identifying the components of a compound or mixture in solution. The aim is to separate each part based on structural differences between them, and hence different functional groups will have other chromatographic properties.
Common types of chromatography include gas chromatography (GC) and liquid chromatography (LC), both of which use a chemical process to separate compounds in a mixture. For GC, the mixture is made up of gases injected into a column filled with an adsorbent material.
The adsorbent material absorbs solvents from the mixture. As a result, each component has different properties and can be separated based on those differences. LC is similar to GC, except it uses a liquid instead of gas and the column contains an absorbent liquid rather than an adsorbent solid.
You may be interested in Enzyme Lactase
How is the Hydroxyl Group Generated?
The organic compounds can be synthesized by using equimolar amounts of the functional groups and anhydride. For instance, oxalic acid and glycolic anhydride react together to form ethanedioic acid on heating in the presence of a catalyst.
Another method to form functional groups is nucleophilic acyl substitution. Here, an electron-rich such as hydroxyl adds to the carbonyl carbon of a carbonyl compound when it attacks it at that position by releasing a leaving group like water. This forms a carboxylic ester. If the -OH group also releases a water molecule during this reaction, it creates alcohol.
What is Carbonyl Group?
A carbonyl group is a functional group. It consists of an oxygen atom with two carbon atoms bonded to it. The carbon atom on the left side of the oxygen atom is also known as the alpha carbon, while that on the right is called the beta carbon, and they are each attached via a double bonyl Group in Structure.
For instance, the structure shown to your right (R-CO-) is a carbonyl group. It has an alpha carbon (the leftmost carbon) and a beta carbon (the rightmost one). Carbonyls are usually classified based on how the oxygen atom bonds to each of the two carbonyl groups in Structure-Based on How Oxygen Bonds to Carbon.
The carbonyl is a significant functional group. It is present in the essential compounds essential for life, including carbohydrates (sugars), amino acids, and proteins.


Carboxylic Acidic Group
A carboxylic acid group is a functional group with the formula R-COOH, where R is an organic residue. Compounds containing this functional group can be referred to as carboxylic acids or carboxylates. They are commonly found in biological systems and form part of it is Phenol Group.


What is the Amine Group?
An amine group is a functional group. It consists of a nitrogen atom with two hydrogen atoms bonded to it. The hydrogen atoms are attached on either side of the nitrogen atom, also known as alpha carbon and beta carbon.
Like hydroxyl groups, amine groups’ presence can influence the properties of a molecule in several ways. For instance, when two amine functional groups are present in a single molecule, their mutual attraction increases the boiling point and decreases the freezing point of those molecules.


What is Aldehyde Group?
The aldehyde group is another fundamental functional group. Aldehydes are involved in many biological reactions, including the oxidation of fatty acids and the glycolysis process. Many compounds contain an aldehyde group as part of their structure, whether they remain aldehyde or are subsequently oxidized to form carboxylic acid groups. The structure of an aldehyde is CH3CHO, and that of a ketone is CH3COCH2CH3.
Aldehydes are also very common in the perfume industry, where they are usually known as top notes in perfumes.


What is Amino Group
Amino groups are functional groups in organic chemistry with the formula -NHR’ or H2N-R’. Amino groups are derived from amines by substitution of hydrogen with an alkyl or aryl group. The central nitrogen atom has two substituent, which may be two alkyls (the “primary amine”).


What is an Ester Functional Group?
An ester group has the structure R-COOR’, where R represents an organic group and R’ represents a hydrogen atom or another organic group. This is one of the essential biomolecules because it is often used to store energy in animals (like fats) and fuel plants.
Esters are formed when alcohol and acid react together. The R’ group is a hydrogen atom in the case of a mono-ester, but it can also be organic groups like carbons or nitrogen atoms.
Esters usually form from carboxylic acids that have a COOH end because the oxygen atom provides extra stability. It is also important to remember that esters are more stable in the presence of water because they bond with it and become solvated.
You may be interested in the Fluid Mosaic Model
How Can You Identify an Ester Functional Group?
You may be able to recognize an ester group because it has properties similar to other common groups like carboxylic acids (ketones) and alcohols (amines).
It is important to remember that esters have a carbonyl group (-C=O), two alkyne groups (C-C triple bond), an oxygen atom, and two other organic functional groups that are R’ and R. Esters are made up of carbon, hydrogen, oxygen, and sometimes nitrogen or sulphur.
The ester is found in a wide variety of foods and beverages. Esters are common in fruits, vegetables, and spices due to their aromatic smells and flavours. Some examples of these esters include ethyl butyrate (cherries), isoamyl acetate (banana), and ethyl acetate (apple cider).
The ester is essential in organic chemistry because it can be found in many different substances. Esters are used to creating fragrances, flavours, and other chemicals in perfumes and colognes. Esters are common in pharmaceuticals like aspirin because of their analgesic, anti-inflammatory, and antipyretic properties (pain reliever, fever reducer). These pharmaceuticals can be used to treat various physical pains related to pain in the muscles or headaches.
How is Ester Formed?
Carboxylic acids will react with alcohols or phenols by becoming esters. This reaction involves a nucleophilic substitution and an elimination of water, which are both mechanisms that must occur together to produce esters.
The first step of this mechanism is when the molecule with the carboxylic acid functional group attracts an electron-rich hydrogen atom by donating its lone pair into a sigma bond. This activates the hydrogen atom and binds with the carboxylic acid group, making it temporarily a carbonyl group.
After this happens, the molecule can split into two separate parts because of the elimination of water through an addition-elimination reaction mechanism. The second step is when another electron-rich hydroxyl comes together with the carbonyl group to form a new C-C triple bond.
This is where monoesters are formed, and you have an ester group with two R groups (like R-COOR’, H2O, or R-COOH).
You may be interested in the Kreb’s Cycle
What is Ester Formula?
Ester formula is the formula of an ester functional group, that is RCOOR’. It can often be found in books, lab notebooks, exams, and handouts during class. With this information, you can also find out what type of esters are common in fruits (esters with butyrate), perfumes (aldehydes), flavours (esters), plastics (phthalates), and pharmaceuticals (aspirin, codeine).


What is the Simplest Ester Molecule?
The simplest ester molecule is methanedioic acid, CH3COOH. Also known as oxoacetic acid, it has a molecular formula of C4H6O2 and a structural formula of HOOC-CH2-COOH.


What is the Simplest Ester Compound?
The simplest ester compound is formic acid, HCOOH. It has a molecular formula of C4H6O2 and a structural formula of HOOC-CO-COOH. With only one CO group, it is also the simplest carboxylic acid.
What Does R Mean in Functional Groups?
R in functional groups means the number of carbons in a compound. For example, R means one carbon (CH3), and pentane means five carbons. So when you see an R group, it means there is carbon with the functional group. So CH3CO-O-CH2CH2OH would be methyl, the first R, and pentanoic acid, or 5-hydroxyvaleric acid would be pentanone, the second R.
What Does Ar Mean in Functional Groups?
Ar stands for aromatic rings that are cycled through less than four carbons atoms. This means that there is only one ring attached to a functional group, multiples of 4. Often you will see this as the first R group in CH3CO-Ar.
What is +R Effect?
The +R effect is that some molecules with a specific group will positively charge in an acidic environment. This means that the pKa of the molecule will be lower than it would be if the molecule did not have this functional group attached to it.
For example, ethanoic acid contains one ester functional group with a structure of H(C=O)-C (CH3)2. The pKa for ethanoic acid is 8.24. This means that at a pH of 8, 24% percent of the molecules will have a positive charge, and 76% percent would negatively charge as the molecule is deprotonated.
Since a negative charge is an equilibrium between protonated and deprotonated states, we can say that hydrogen ions neutralize 76% of the molecules to give H (CH3COO)-H+.
At the pH of 4, 24% percent of neutralized ethanoic acid molecules will have a positive charge. This is called the +R effect because if you know an ester has this functional group, it can give off a positive charge instead of negatively charged in an acidic environment.
You may be interested in Meristematic Tissues
What is -R Effect?
The -R effect shows what happens when there is a negative charge in an alkaline environment. So instead of the pKa being lower than if there were no functional group, it would be higher. This means that more molecules are protonated rather than deprotonated.
For example, let’s take pentanoic acid as an example. The pKa for this molecule has a value of 1.88, which is less than the pKa for ethanoic acid (8.24).
This means that under acidic conditions, more molecules are deprotonated and carried away by H+. In an alkaline environment, there will be more molecules protonated or positively charged.
How Do You Explain +R Effect and -R Effect?
The +R effect is when there are lone pairs of electrons on an atom in a molecule that can donate hydrogen ions, and the positive charge will be repelled away from the carbon (C) or nitrogen (N) atom.
This means that more protonated molecules (a hydrogen ion will be taken from a water molecule and given to the protonated molecules, forcing it into solution) than there would be without the lone pair of electrons.
It also shows the difference between an ester with one R group as compared to no R groups.
The lone pair of electrons on the oxygen is donating hydrogen ions to all of the molecules, protonating more than half of them. This makes ethanoic acid have a lower pKa (8.24) than pentanoic acid (1.88).
The R and R mean that there is a four-membered ring with two substituents attached to it. So CH3CO-R would be methyl, the first R, cyclopropane carboxylic acid, or 2-cyclopropane carboxylic acid, which would be another example of where you see an R. Acid names have the COOH functional group on them, and a lot of times, these substituents are alkyl groups.
What is the Priority Order of Functional Groups?
The priority order is O, N, S. O comes first because the most electronegative elements get to be on the outside of the molecule that can donate electrons and lone pairs best. Then you go down the triangle.
It corresponds with N being ranked 3 in electronegativity (compared to fluorine and oxygen) and being in the middle so that N gets to be more on the inside and participate better in reactions. S is last because it has no lone pairs of electrons to donate or share, so it is always outside a molecule.
You may be interested in Oxidative Phosphorylation
Conclusion
There are many types of organic functional groups that can be found in molecules. They all have different properties, but they are classified according to their chemical reactivity and acidity. The hydroxyl group is the simplest of these common organic functional groups. It consists of a hydrogen atom attached to an oxygen atom with two distinct properties- its acidity (which can be measured by its pK value) and nucleophilicity, or how reactive it is with other molecules.
The seven broad categories include the hydroxyl group, carbonyl group, carboxylic acid group, amine group, amino acids/amino acids, aldehydes, and ketones.
I hope that this article has helped you understand these types of molecules better and how they work. In case that assignment is still confusing, our professional writers are ready to help.