Enzyme Lactase- Definition, Function, & Location/Production
Introduction
The enzyme lactase is an enzyme that is produced naturally in humans to break down lactose. The name comes from the Latin word “lac,” meaning milk, and the Greek word “sucrase,” meaning sugar. Lactase is usually produced during lactation, but production decreases at weaning and finally ceases at the end.
However, studies have shown that if a mother’s diet includes raw milk or other dairy products (such as cheese), then the lactase enzyme activity can continue for a few years after weaning.
The enzyme lactase is a member of glycoside hydrolases family 13, and it’s the gene LCT (lactase) involved with this specific enzyme. The greatest amount of lactase is found in infants and decreases over time, finally ceasing to an adult level at around 5 years old.
Functions of Enzyme Lactase
The following are the main functions of enzyme lactase:
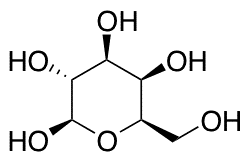
- Lactase is essential in breaking down the disaccharide called lactose. Lactose is present in milk and is broken down into two monosaccharides, glucose, and galactose.
- Enzyme lactase has a glucose-galactose bond which cleaves to produce glucose and galactose.
- In addition, lactase has a vital role in the body converting excess glucose into glycogen. When lactose is present, and the body cannot break it down, glucose is used to generate energy.
- Lactase also plays a crucial role in the maintenance of mucosal surfaces in the human body.
- Commercially, the enzyme lactase is used for the production of lactic acid, cheese, and yogurt.
- Lactase extracted from certain yeast is used to supplement infant formula and other food products to reduce lactose.
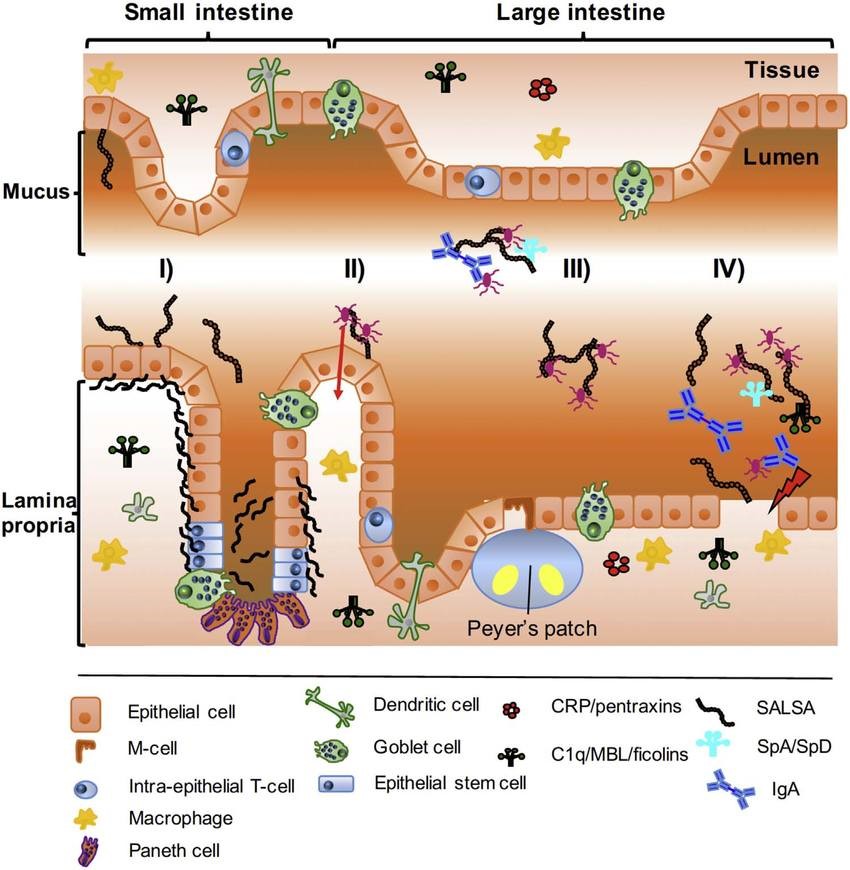

Where is Enzyme Lactase found?
Human lactase is found in various tissues, including the small intestine and colon, and is in two forms: secreted and membrane-bound.
Membrane-bound lactase is found on the surface of epithelial cells in the brush border of the intestinal mucosa. The exocrine secreted form is found in acini as well as on mononuclear cells and lamina propria.
Enzyme lactase may also be present at low levels in other organs and tissues, including the spleen and liver.
Some lactase activity has been found in human reproductive organs, including the testes. It appears to aid spermatozoa maturation and survival during transit through the epididymis.


How is Enzyme Lactase Produced?
The enzyme lactase is produced naturally in humans to break down lactose. The lactase gene LCT (lactase) is found on chromosome 2 in humans. In mammals such as horses and humans, the enzyme lactase is produced first in infants.
During breastfeeding, around 75% of the lactose ingested by mother and baby will be broken down into glucose and galactose by lactase resident in the infant’s intestine.
Lactase is synthesized in the Golgi apparatus and packaged into secretory vesicles with Golgi’s membrane. It is then transported to the apical membrane of the intestinal cells.
Supplements of lactase are available for adult humans who still lack this enzyme and suffer from lactose intolerance.
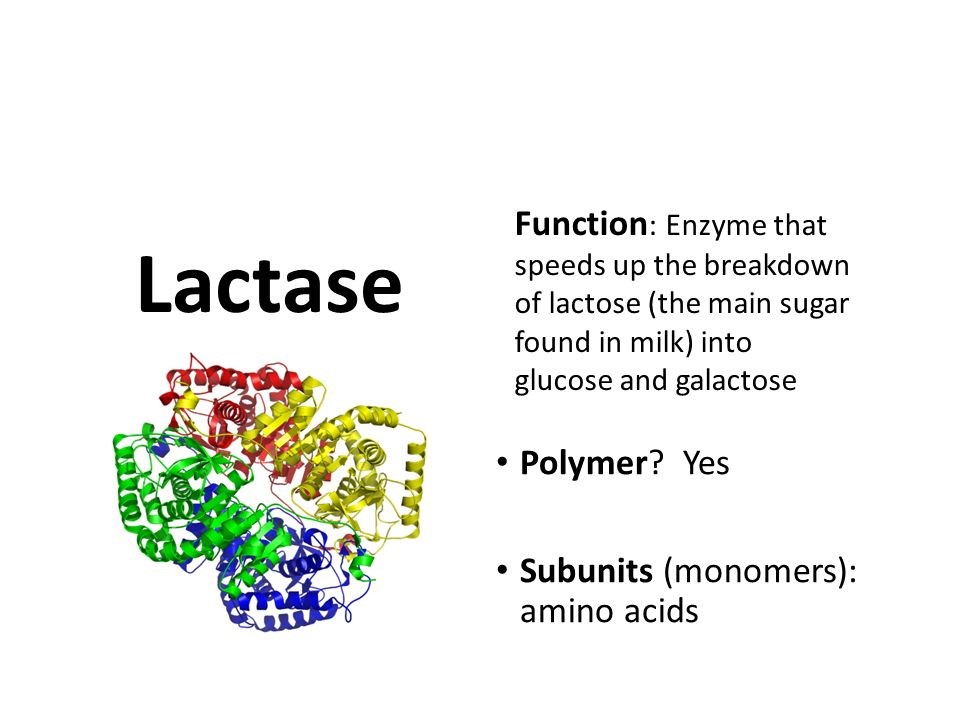
Lactase Structure

The enzyme lactase is a glycopeptide containing six identical subunits. The structure of lactase is very similar to that of sucrase. The enzyme’s primary structure consists of two domains connected by a short linker region. This structure is the smallest of α-glycosidase enzymes that hydrolyze O-glycosyl compounds.
The lactase enzyme contains six identical subunits arranged in a circle or ring-like structure. The six identical subunits are arranged around a central cavity that contains the catalytic residue Asp102.
The lactase gene is made up of three exons and two introns. The initial amino acid chain that produces enzyme lactase is called pre-pro-lactase.
The pro-form can then be processed by an enzyme called signal peptidase to produce the final active form of lactase. The human gene is known as LCT, located on chromosome two.
The enzyme lactase is made from a single polypeptide that is approximately 1927 amino acids long. The enzyme comprises four identical subunits arranged in a circle and linked together by ten types of disulfide bonds.
Lactose Intolerance


Lactose intolerance is the inability to digest lactose, a sugar found in milk. It is also called hypolactasia. Symptoms include diarrhea, bloating, and abdominal pain, which may be mild and occasional to severe. Lactose intolerant people may also experience nausea.
Causes of Lactose Intolerance
Lactose intolerance is a condition that develops due to insufficient levels of lactase enzyme in the gut, which breaks down lactose into glucose and galactose. Several factors can cause lactose intolerance. These include:
- Age.
- Genetics. It is an inherited condition and becomes more of a problem after weaning in adulthood; also, many genes involved in lactose intolerance have been identified.
- Gastrointestinal conditions like coeliac disease, Crohn’s disease, and ulcerative colitis.
- Drugs/toxins that kill gut bacteria like antibiotics and anti-malarial medicines.
- Infections like rotavirus, Giardia lamblia, and viral infections.
- Stress and emotional factors that affect the gut lining like surgery, gastrointestinal disease, and infections.
Treatment of Lactose Intolerance
Lactose intolerant people can tolerate small amounts of lactose. Excess lactose can be tolerated when consumed in small quantities for some time.
Eating dairy products containing smaller amounts of lactose in one sitting will help lactose intolerant people tolerate it.
There are supplements available for lactose-intolerant people containing the enzyme lactase added to dairy products. These supplements facilitate the breakdown the lactose into glucose and galactose. The process helps lactose intolerant people to absorb glucose and galactose without getting any symptoms.
Factors to be considered during lactose intolerance treatment are:
- The type of dairy product consumed like milk, cheese, and yogurt contains different amounts of lactose.
- Amount of dairy products consumed at one time.
- The period between lactose intake and symptoms.
- Genetic composition of the patient
- The amount of lactose that is in a food product
Types of Lactose Intolerance
Lactose intolerance is divided into primary, secondary, and congenital.
Primary Intolerance
Primary lactose intolerance is the inability to break down lactose in adulthood after weaning. The condition is genetically determined and appears at any time from childhood to adulthood. People with primary lactose intolerance can digest the sugars in milk and other dairy products when they are taken in small amounts.
Secondary Intolerance
Secondary hypolactasia occurs if there is an underlying disease that damages the lining of the gut. This lowers lactase production but will return to normal when the condition is treated and healed. The most common disease that is associated with secondary lactose intolerance is coeliac disease.
Congenital Lactose Intolerance
Congenital lactose intolerance occurs if an individual is born without the enzyme lactase to break down lactose. It is a rare condition but is mainly seen in Asian and African populations.
You may be interested in Evolution History and Relationships
Summary
Lactase is an enzyme that breaks down lactose, the sugar in milk. It’s found in human saliva and helps to digest dairy products. If you’re suffering from a condition called lactose intolerance, this means your body doesn’t produce enough of the enzyme needed to break down all the sugars present in dairy foods.
Symptoms of lactose intolerance include abdominal cramps, diarrhea, gas (flatulence), nausea, and other unpleasant symptoms such as bloating or constipation, depending on how much food was eaten containing lactose sugar.
As people age, their bodies may not be able to make enough enzymes, which can lead to serious health consequences.


I‘m a freelance content and SEO writer with a passion for finding the perfect combination of words to capture attention and express a message. I create catchy, SEO-friendly content for websites, blogs, articles, and social media. My experience spans many industries, including health and wellness, technology, education, business, and lifestyle. My clients appreciate my ability to craft compelling stories that engage their target audience, but also help to improve their website’s search engine rankings. I’m also an avid learner and stay up to date on the latest SEO trends. I enjoy exploring new places and reading up on the latest marketing and SEO strategies in my free time.